- Nitrogen-filled tires: myths and reality
- Analysis: Algae for carbon dioxide (CO2) capture
- Synfuels (CTL, OTL, GTL, BTL, XTL) Round-Up
- Bioethanol: gasification and biofermentation
- Underground Coal Gasification: Keep the coal in the ground, convert it to gas
- Analysis: Algae for CO2 capture - II
- Comments: Marine cement production process
- Analysis: How much biodiesel will jatropha cultivation in UP produce?
- Flue gas or Fuel ? : India's CTL Dilemma
- Typical yields from algal biofuel technologies
- News: BP and Ergo Exergy agreement on UCG
- Opinion: Oil Prices Depend on More on Speculation than assumed previously
- Bauxite vs beliefs in India: The case of Sterlite & the Dongria Kondh
- Sustainability and Cement CO2 emissions: US cement outlook
- What effects will the current economic downturn have on carbon trading and GHG legislation?
- Resource Conservation & Game Theory
- Redwoods vs solar panels
Dec 30, 2008
Popular posts of 2008
Dec 25, 2008
Happy Holidays!
Update: My article on The Big Biofuels Blog is: U.S. Biofuels : Near-term challenges and prospects
Dec 19, 2008
X-Algae: Mutant algae for biofuel production?

"Abstract: Research seeks to alter the optical characteristics of microalgae in order to improve solar-to-biofuels energy conversion efficiency in mass culture under bright sunlight conditions. This objective is achieved by genetically truncating the size of the light-harvesting chlorophyll arrays that serve to absorb sunlight in the photosynthetic apparatus."
Nature optimizes each algae to maximize its light absorption to survive in the wild. However, the large size of these light-absorbing chlorophyll arrays leads to sub-optimal light utilization when growing algae for biofuel production, because light has to be distributed as far as possible in the growth medium to ensure optimal light utilization and increased yields per unit time per unit area. When grown in the mass culture, the mutant algae evolved oxygen at a 2 to 3-fold higher rate compared to the wild-unmodified algae, indicating potential algal biofuel yield increases of 100% to 200%.
Implications for algal biofuel production:
Previous posts (yields, CO2 capture, economics) on this blog have focused on various aspects of algal biofuel production. Because algal yields significantly influence the economics, increasing the light absorption per unit volume in the algal growth medium would lead to accelerated commercialization.
Labels: algae biodiesel, algal biofuels, chlorophyll, co2 mitigation, mutant, mutation, process economics
Dec 18, 2008
Opinion: Are win-win solutions to our energy and environmental problems possible?
 |
Labels: environmental economics, global warming, green jobs, renewable energy
Dec 8, 2008
Water, water everywhere.....
- The IPCC Technical Paper VI projects that:
- The per capita availability of fresh water in India will drop from 1820 m3 currently to 1000 m3 (yearly basis) as a result of population growth and climate change. In comparison, the global average by 2025 is expected to be ~5000 m3. India's population will therefore be using 1/5th of the world average per capita water consumption.
- More intense precipitation in Asia would result in a higher runoff and reduction in the portion recharging the groundwater aquifers.
- Agricultural irrigation demands in arid and semi-arid regions of east Asia would be expected to increase by 10% for a 1 degree C increase in temperature.
- Changes in snow and glacier melt will cause seasonal shortages and affect 1/4th of China's population and hundreds of millions of India's population. (~0.3-0.6 billion combined).
- Arid and semi-arid land in Africa would increase 5-8% by 2080.
- Current water stress in Africa will likely be increased by climate change.
- Any changes in the primary production of large lakes (Lake Chad, Lake Tanganyika) will have important impacts on local food supplies.
My perspectives: Economic water scarcity is another dimension of the "Water, water everywhere.." problem. Low-cost means to treat water and responsible aquifer management are required to overcome economic water scarcity. Physical water scarcity will need similar measures, and various end-users (farmers, industry, households) must be encouraged to conserve and recycle where possible. Farm subsidies for water-intensive crops (ex: sugarcane, paddy), will likely have significant impacts on water conservation and scarcity. Balanced policy planning is therefore required to manage local, regional and national water resources. Finally, regional cooperation, as outlined in an earlier post will be necessary to ensure equitable distribution of water resources among different stakeholders.
Related articles:
- A cover story on water scarcity in the October issue of C&EN.
- "Telescopic" rates for high-volume water users in Mumbai.
- My article on resource conservation and a possible role for game theory.
Labels: China, India, IPCC, resource conservation, scarcity, South Asia, sub-Saharan Africa, water
Dec 4, 2008
Graphic of the week: U.S. CO2 sources and regional cap-and-trade agreements
Here is a map of U.S. CO2 sources (from the NETL carbon sequestration atlas) overlaid with the states which are participants/observers in) various regional GHG reduction initiatives. More information from the Pew Center on Global Climate Change. Briefly, the abbreviations in the figure are:
- WCI: Western Climate Initiative
- MGGA: Midwestern Greenhouse Gas Reduction Accord
- RGGI: Regional Greenhouse Gas Initiative
Note: Some provinces of Canada also participate in these agreements, however, they are not shown here.
Labels: carbon tradiing, climate change, co2 mitigation, Pew Center, U.S.
Dec 3, 2008
2007 US greenhouse gas emissions: cement, limestone, natural gas, and other industries
 The Energy Information Administration (EIA) has released a report on U.S. greenhouse gas (GHG) emissions in 2007 (Note: ftp link).
The Energy Information Administration (EIA) has released a report on U.S. greenhouse gas (GHG) emissions in 2007 (Note: ftp link).What attracted my attention was the "other sources" category, of which cement contributed ~46 million metric tons (MMT) of CO2/year in 2007 (~0.8% of total U.S. greenhouse gas emissions). The next highest contributor was lime-making, which involves limestone calcination. Aluminium (3.8 MMT) production contributes much lesser to U.S. GHG emissions compared to cement production. Additionally, iron and steel production likely contributes ~45 MMT CO2/year. On the other hand, recent high-gas prices partly contributed to higher natural gas production (and higher CO2 co-produced from natural gas). CO2 emissions from natural gas-flaring in the U.S. were not projected to change from 2006 (7.8 MMT CO2/year). In the figure, the values for natural gas show a spike in 1995, from my data it appears to be due to significantly higher natural gas flaring, than CO2 production from natural gas.
Labels: aluminum, cement, co2 mitigation, EIA, gas flaring, GHG emissions, iron and steel, natural gas, U.S.
Nov 24, 2008
Sustainability and Cement CO2 emissions: US cement outlook
Instructions to view the mindmap: This mindmap is hosted on mappio.com. Click on the image below to view the mindmap. You must enable flash for the website to do this. On mappio.com, click on the "View Fullscreen" link to get rid of the ads on the right hand side and view the map. You can zoom by using your middle mouse button, click anywhere on the map to fold and unfold links.
Disclaimer: Mappio.com is a free site to upload and share mindmaps, I am not affiliated with it.
A summary of the mindmap follows:
Approximately ~1.3 T CO2 are produced per T of cement. Globally, the cement industry contributes to 5% of the anthropogenic CO2 emissions (1.34 giga tons of CO2).
Comparisons between cement production and pulverized-coal combustion:
There are some commonalities and contrasts between CO2 emissions from coal-fired power plants and cement plants. Both "commonly" consume coal as a fuel,and flue gas emitted from both processes is relatively dilute in CO2. However, a greater share of CO2 emissions from cement are a result not of coal burning, but due to the calcination of the raw material (calcium carbonate, CaCO3). The operating temperature of a cement kiln affects the quality of the clinker and the cement produced, and therefore controlling it is relatively more complex compared to coal-fired combustion. Additionally, cement production is essentially a hot gas-solid heat exchange process (with simultaneous mass transfer, chemical reaction and material flows) whereas in coal combustion, the objective is to produce a hot flue gas stream to generate steam.
What has the cement industry done to reduce its CO2 emissions?
In cement production (similar to heavy-metal industries such as bauxite and steel) energy efficiency (lower CO2 emissions) directly translate to cost savings. Therefore, the industry has an incentive to maximize the tons of product produced/unit of energy spent. Innovations in kiln design (preheaters, precalciners) as well as a move away from wet process kiln technology have resulted in considerable energy savings. Because a greater portion of the CO2 emissions from cement plants are from the raw material calcination itself, any process that requires lesser raw material to be calcined significantly reduces the CO2 emissions from (and energy requirements of) cement production. Examples of this are blending pozzolonas (fly ash) to cement and blending ground limestone into cement. The ASTM standard limits fly ash blending at 25% w/w in cement for reinforced cement concrete applications, whereas the relevant standard for limestone currently allows 5% blending. Because both of them displace an equivalent amount of clinker, blending limestone or fly ash results in reduction of CO2 emissions and is also very cost-effective. However, recent NOx regulations have forced powerplants to reduce flame combustion temperatures to avoid high-temperature NOx, and this results in residual carbon in flyash. High-carbon fly ash is not suited to be blended into Portland cement. Fly ash blending gives durability to cement. Overall, in my opinion, cement manufacture is a nice example for industrial ecology, because fly ash (a byproduct of coal combustion) and gypsum (a by-product of phosphoric acid manufacture) are used to make cement, which in turn is used to make concrete.
What can the industry do in the near future?
A few of the many innovations in reducing GHG emissions from cement production include:
From a general perspective, the process of precipitating calcium carbonate from CO2-saturated solutions requires high-pH conditions. This scenario is a catch-22 situation, because increased CO2 concentrations in water (under high pressures) which is essential for precipitating CaCO3 (for example), also results in decreased pH, which disfavors carbonate precipitation. On the other hand, many marine organisms such as corals, mollusks and algae form CaCO3 either within their bodies or externally, under relatively dilute-calcium concentrations. Some alkaline materials that could be added to water to increase its pH economically are: alkaline fly ashes, and cement kiln dust.
Another example of a company involved in making carbonates is Carbon Sense Solutions Their process involves accelerated CO2-curing of concrete, which is essentially a reverse of the calcination step. I partly commented on this process on the peakoil forum At best, thsi process would make concrete (and cement production) carbon-neutral. However, getting a CO2-source close to the curing plant would require additional infrastructure to transport CO2. Compared to this, the use of external cations (either from sea water (Calera) or an added alkaline material) has the potential to result in a net-reduction of CO2 emissions
Summary and Outlook:
In the short-term, processes which utilize CO2 would provide low-cost CO2 offsets to cement producers, whereas the long-term approach likely involves developing the infrastructure for low-cost carbon capture and storage (CCS).
Labels: Air Products, algal biofuels, Cal Star, Calera, Carbon Sense Solutions, cement, co2 capture, CO2 Solutions, CO2 to fuels, concrete, fly ash, GreenFuel, ITM, limestone, RGGI, sustainability, WCI
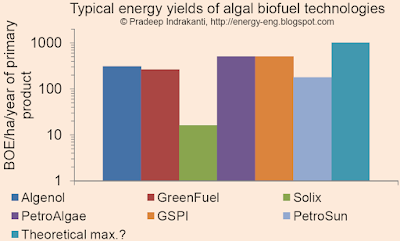
Typical yields from algal biofuel technologies
This is an examination of yields of "primary product" (algal oil/ethanol/biodiesel), expressed as barrels of oil equivalent/hectare of land area/year, from various algal (algae) biofuel technologies. I used data from company websites and press releases and converted the algal oil/ethanol production to a BOE/ha/year basis.
From the above figure, typical "yields" range from 100-1000 BOE/ha/yr. Compare this to Dr. Benemann's recent statement that the maximum algal yield without using genetically modified algae would be ~2000 gal algal oil/acre/year (101 BOE/ha/year).
Disclaimer: This is not meant to be a comparison of various processes or an endorsement/critique of a specific process. Utilization of and the value for the algal biomass and the biofuel determines the overall process economics. My assumptions and data are given below:
- Algenol: 6000 gal EtOH/acre/year
- Solix data from here.
- GreenFuel, from a previous post
- PetroAlgae: Assumed 200x current soybean oil yields (200x50 gal oil/acre/year).
- GSPI: Link here
- Theoretical maximum: from CircleBio's website, assuming 20,000 gal biodiesel worthy plant oil/acre/year.
- I further assumed that 1 T of algae oil gives 1 T of biodiesel, unless mentioned otherwise on the company's website (the ratio is ~0.96 for soy oil).
- Calorific value of 33MJ/L for biodiesel and 20 MJ/L for ethanol.
Related posts:
Analysis: Algae for CO2 capture - II
Analysis: Algae for carbon dioxide (CO2) capture
Labels: algae, algae biodiesel, algal biofuels, climate change, co2 mitigation, CO2 utilization
Nov 9, 2008
Analysis: Algae for CO2 capture - II
Capital expenditure: 92 million $, CO2 fixed: 50,000 T/year (2011).
Algal oil production: 1.3 million gal/year.
Cost algal oil: 4 $/gal.
Price of CO2 offsets: 20 $/T CO2.
Timeline considered for IRR calculations: 10 years.
The rest of the scenarios are explained in the figure. Doubling the yields (and CO2 captured) does increase the IRR and lower the payback periods more than doubling the oil prices (mentioned in my last post). Moreover, CO2 trading plays only a minor role by itself, but results in higher IRRs and lower payback periods when considered along with other possibilities. The highest IRR and lowest payback occur when both yields as well as the oil prices are significantly higher than in the base case scenario.
Labels: algae, algal biofuels, co2 mitigation, CO2 to fuels, CO2 utilization, economic evaluation, process economics
Nov 7, 2008
Comments: Marine cement production process

Recently, Calera proposed a process suitable for power plants and other major CO2 emitters which converts CO2 to calcium carbonate (CaCO3). From the Scientific American:
....Calera hopes to get over that hurdle quickly by first offering a blend of its carbon-storing cement and Portland cement, which would not initially store any extra greenhouse gases but would at least balance out the emissions from making the traditional mortar. "It's just a little better than carbon neutral," notes Constantz, who will make his case to the industry at large at the World of Concrete trade fair in February. "That alone is a huge step forward.".."
In the following, I discuss some of the critical challenges to make this a feasible operation. The roles of the abundance of calcium and magnesium in sea water and its pH are discussed in detail. The formation of calcium/magnesium carbonates from sea water requires energy to proceed. This energy could be supplied either in the form of a pH shift (by adding strong base) or by coupling the carbonate formation to other processes (algal photosynthesis). Why does blending calcium carbonate in cement make economic sense? The unit price for medium ground (4-9 um) CaCO3 is around 95-100 $/T whereas portland cement retails at ~180 $/T . Each ton of CaCO3 blended will save 80 $.
Sea water has 0.01 gram-mol Ca2+/kg-sea water and 0.05 gram-mol Mg2+/kg-sea water . The precipitation chemistry of calcium and magnesium carbonates is complicated, involving many minerals. If we precipitate all of the Ca2+ and Mg2+ as carbonates (CaCO3 and MgCO3), sequestering 1 T of CO2 would require 360 T of sea water. Additionally, carbonate precipitation does not occur below a pH of ~10, whereas sea water has a pH of ~7.5-8.4 , which could be decreased by increasing the partial pressure of CO2. Increasing the pH of the solution to favor carbonate precipitation likely requires the use of a base, such as sodium hydroxide, NaOH.
Apart from the large volumes of sea water to be handled to make carbonates, the manufacture of NaOH involves the electrolysis of brine and is fairly energy-intensive, producing 4.6 T CO2/T NaOH. Depending on the extent of NaOH requirements, this could represent a significant source of CO2 emissions. (Therefore, using NaOH to sequester CO2 does not make much sense).
Switching gears to learning from nature, certain coralline algae form CaCO3 from sea water. This CaCO3 acts as a binder, stabilizing the coral reef. The mechanisms involved are fairly complex for my understanding, but an essential process is the equilibrium between the bicarbonate and carbonate ions, depending on the pH.
pH = 10.33 - log([HCO3-]/[CO32-])
Under sunlight, algae photosynthesize dissolved CO2 or HCO3- to form organic compounds (CH2O). This leads to an increase in the local pH. An example of how this happens is given in a paper by John Dodson (subscription required). This increased pH combined with the presence of ion-selective membranes, which admit only certain ions might be how algae use photosynthesis to drive an endothermic CaCO3 precipitation reaction.
Conclusions:
- Large volumes of sea water are required to sequester CO2 as calcium/magnesium carbonates. Membrane separations will likely play a critical role in reducing this requirement.
- On the other hand, CO2 sequestration in brines (by product of oil and gas production) also has been investigated. The advantage here is that these brines have high concentrations of calcium, iron and magnesium ions. Therefore, potentially less volumes of brine are needed compared to sea water to sequester the same amount of CO2. The obvious problem here is to increase the pH of the solution to precipitate dissolved CO2 as carbonate. Instead of using a strong base such as sodium hydroxide, folks at NETL used residues from bauxite (an ore of aluminium) processing to buffer the solution pH.
- One needs a driver to precipitate calcium/magnesium carbonates from sea water. In the chemical process described above, it is the addition of sodium hydroxide.
- The smarter way nature has found around that requirement is the formation of carbonates via the bicarbonate<->carbonate equilibrium, shifted towards the right by algal photosynthesis (a reaction converting bicarbonate/CO2 to organic carbon using sunlight, thereby increasing the pH).
Labels: algae, calcium carbonate, cement, co2 mitigation, magnesium carbonate
Nov 3, 2008
Site visits to the Energy Engineering Blog
Oct 30, 2008
CO2 to fuels processes - II
"Dr. Naveed Aslam, inventor of the company's technology and chief technology advisor, commented: "Unlike other CO2 to fuel approaches, Carbon Sciences' technology does not use molecular hydrogen (H2) because the creation and reaction of H2 is very energy intensive. Rather, the company's approach is based on a low energy biocatalytic hydrolysis process where water molecules (H2O) are split into hydrogen atoms (H) and hydroxide ions (OH) using a biocatalyst. The hydrogen atoms (H) are immediately used in the production of hydrocarbons and the free electrons in OH are used to power the various biocatalytic processes." "Our technology is not based on photosynthetic plants where sun light is used to drive biofuel production reactions, such as in algae. Instead, it is based on natural organic chemistry processes that occur in all living organisms where carbon atoms, extracted from CO2, and hydrogen atoms extracted from H2O, are combined to create hydrocarbon molecules using biocatalysts and small amounts of energy. Our innovative technology allows this process to occur on a very large industrial scale through advance nano-engineering of the biocatalysts and highly efficient process design," concluded Dr. Aslam."
My opinions given below:
Understandably Carbon Sciences is justified in not fully revealing the details . However, the splitting of water to produce protons (H+) and hydroxide ions (OH-) still consumes energy. All the biocatalyst does is to speed up this transformation. It cannot influence the thermodynamics (feasibility) of this reaction. Judging by what the release says, I think that there is a sacrificial oxidant (something which gets oxidized, ex: simple sugars, providing the energy to drive the splitting of water) involved.
Related links:
Opinion: CO2 to fuels processes
Carbon Sciences Announces Prototype Plan for CO2-to-Fuel Technology
Labels: Carbon Sciences, co2 mitigation, CO2 to fuels, CO2 utilization, hydrogen
Oct 22, 2008
PBS Frontline: Heat
Labels: biofuels, biofuels global warming Science fossil fuels, carbon capture, carbon tradiing, co2 mitigation, Frontline, PBS, solar photovoltaics
Oct 21, 2008
Analysis: Algae for carbon dioxide (CO2) capture
This post describes a simplified economic analysis of an algal biofuel technology that converts carbon dioxide (CO2) from cement plants into (potentially) useful algal oil. I examined various key factors such as CO2 offset price, price of algal oil, and productivity that affect the profitability of such a process.
Based on my analysis I conclude that the single most important factor that affects the economics of CO2 capture is the algal biomass yield (mass produced/unit area). Doubling the productivity (and the CO2 offset) per hectare decreases the payback time by 50 % (15 years to 7 years).
Disclaimer: This is not a critique of the specific algal biofuels process proposed. CO2 mitigation using algae is one of the answers to our grand energy challenges, and we must continue to address these issues.
Assumptions:
The Holcim plant in Jerez likely produces a fraction of the total 5.1 million tonnes of cement per annum (5.1 MTPA). (A cement plant in India I worked at produced 2.6 MTPA, and it was the largest in Asia at that time. Not having first-hand data for this specific facility, lets assume that this plant is 1 MTPA, for the sake of comparison. The exact production does not alter the results significantly).
Cost of algal oil: 4 $/gal
Price of carbon offsets: 15 Euros/T CO2 (20 USD/T CO2)
(A high-cost scenario for algal oil and carbon offsets would be 6$/gal, 50 $/T CO2. This is also addressed in the analysis.)
Data:
Each lb of cement produces 1.0 lb of CO2 (U.S. average, pg.10).
Total CO2 to be mitigated annually by 2011: 50,000 T in 100 ha (0.05 MTPA CO2 in 100 ha) .
Algal biofuel production: 1.3 million gallons/year
Calculations:
Total CO2 production: 1 MTPA
By 2011, the 100 ha. facility would mitigate 50,000 T of CO2 (0.05 MTPA CO2). This would be 5% of the CO2 emissions (if the Jarez facility production is 1 MTPA)
Average CO2 use of algae: 0.0005 MTPA/ha.
Revenues from algal biofuel: 5.2 million $/year
Revenues from carbon offsets: 1 million $/year
Total revenues: ~6 million $/year
Results
Capital cost of algal facility: $ 92 million/0.05 MTPA CO2.
Area needed for 5% of the cement plant's CO2 output (assuming 1 MTPA production): 543 U.S. football fields (5.4 football fields = 1 ha.)
Payback on investment: 92/6 =~ 15 years. In comparison, typical payback for a new chemical plant is ~ 7 years.
Higher CO2 prices (50 $/T CO2), decrease the payback period by ~ 3 years. Higher algal oil prices (6 $/gal) and 20 $/T CO2 prices will result in a payback period of approximately 11 years.
Higher oil and higher CO2 prices will lower this period (11 yrs) by an additional ~2 years. However, doubling the productivity (and the CO2 offset) per hectare decreases the payback time by 50 % (15 years to 7 years).
The revenues from algal biodiesel + carbon offsets will be partly offset by the parasitic losses from the power plant to run the system. I don't have a feel for how much these utility costs would be. Any comments, anybody?
Bottomline The single most important factor that affects the economics is the productivity of algal biomass/unit area. Doubling the productivity (and the CO2 offset) per hectare decreases the payback time by 50 % (15 years to 7 years).
Labels: algal biofuels, biofuels, co2 mitigation, CO2 utilization, GreenFuel
Oct 17, 2008
Synfuels (CTL, OTL, GTL, BTL, XTL) Round-Up
 Given below is a compilation of the latest news, analyses and resources on synthetic fuels from hydrocarbons (coal-to-liquids, biomass-to-liquids, gas-to-liquids, oil sands-to-liquids)
Given below is a compilation of the latest news, analyses and resources on synthetic fuels from hydrocarbons (coal-to-liquids, biomass-to-liquids, gas-to-liquids, oil sands-to-liquids) Analyses
The Impacts of Synfuels (CTL,GTL, BTL, OTL) on World Petroleum Supply
RAND Study Concludes Oil Sands Synthetic Crude Can Be Cost-Competitive with Conventional Petroleum Even Over a Wide Range of CO2 Prices
New Life-Cycle Analysis Concludes Neither GTL or CTL a “Reasonable Path” for Energy Security With Reduced GHG Emissions
Study Suggests “Flexible Carbon to Liquid” Fuel Process Could Displace 15-20% of Transportation Fuels in the US
The tale of two synthetic fuels & Using champagne to make beer
News
Australia:
Linc Energy Begins Producing GTL Liquids from Underground Gasification Syngas
South Africa:
Biofuels singled out as 'best option' for alternative fuels in SA
USA:
Synfuels Converts Natural Gas to Gasoline to Cash
New Route to Hydrocarbon Biofuels: A simple catalytic process converts plant sugars into gasoline, diesel, and jet fuel.
Synthesis Energy Systems Options Up to 15 Methanol-to-Gasoline Technology Licenses for Coal-to-Gasoline Projects
Researchers Propose Dual-Bed Configuration to Increase Efficiency and Reduce Emissions from Coal Gasification
China:
Shenhua Ningxia Coal Group boosts CTL project with Sasol
Is it the end of the line for coal-to-oil in China?
India:
Sasol mulls dlrs 8bn India CTL plant
UK:
Small Scale FT Contract with Thai National Oil Company
Resources:
Diesel Fuel from Bolivian Natural Gas by Fischer-Tropsch Synthesis using Nitrogen-rich Syngas
DOE Releases Feasibility Study for Small-Scale Conceptual Coal-to-Liquids Facility in Appalachian Basin : Technical and Economic Assessment of Small-Scale Fischer-Tropsch Liquids Facilities
An Engineering-Economic Analysis of Syngas Storage
Small-Scale Fischer-Tropsch
Labels: ACS, Australia, biomass to liquids, BTL, China, coal to liquids, CTL, Fischer-Tropsch, GTL, India, Linc Energy, oil sands, OTL, RAND, Sasol, syntroleum, UCG, underground coal gasification
Oct 15, 2008
News: Cleaner technologies for coal at Penn State
 Structural representation of a South African intertinite-rich Highveld coal. Carbon atoms are green, oxygen atoms are red, and sulfur atoms yellow. Courtesy of Daniel van Niekirk / Jonathan Mathews
Structural representation of a South African intertinite-rich Highveld coal. Carbon atoms are green, oxygen atoms are red, and sulfur atoms yellow. Courtesy of Daniel van Niekirk / Jonathan Mathews
- Direct liquefaction of coal to produce jet fuels (JP-900).
- Better molecular models for coal, CO2 sequestration in coal seams.
- Understanding coal reactions using femtochemistry.
- Adapting existing refineries for coal conversion.
- Making more comprehensive use of coal, producing value-added compounds.
- Molecular-basket adsorbents to capture CO2 from flue gas streams.
Labels: clean coal technologies, CO2 sequestration, coke, direct liquefaction, jet fuels, molecular modeling, molecular-basket adsorbents, Penn State
Oct 13, 2008
The tale of two synthetic fuels & Using champagne to make beer
I provide a brief description of two processes to produce synthetic fuels, coal-to-liquids (CTL) processes and synthetic crude oil (SCO) from tar/oil sands. The economics behind SCO and CTL production are briefly discussed. One of the critical factors influencing lifecycle CO2 emissions from and economics of the CTL and SCO processes is the C/H ratio of the original fuel source (tar sands/coal). The findings of a recent RAND report (Unconventional Fossil-Based Fuels Economic and Environmental Trade-Offs) are discussed from this perspective.
Overview of the process economics of SCO from oil sands:
Canada has the world's second largest reserves (179 billion barrels) of proven oil, of which >95% comprise oil sands. About 80% of Canada's oil sands deposits are too deep below the surface to use open-pit mining. These deep deposits have to be processed in situ using techniques such as steam-assisted gravity drainage (SAGD). The rest can be accessed via open-pit mining techniques.
Surface (open-pit) mining is a material-intensive operation, with 1.6 T of tar sands to be handled per barrel of SCO produced. The water requirements are also high. It is also fairly energy-intensive, consuming 1 barrel of natural gas equivalent of energy to produce 8 barrels of SCO. For both in situ as well as surface processes, the overall energy requirements (including mining, extraction, coking, and hydrotreating) are approximately 40-45 % of the calorific value of the syncrude. This heat is mainly supplied by natural gas. The price/unit of energy for crude oil is much higher than that for natural gas, and this price differential drives the economics of the process for extracting and upgrading the bitumen from tar/oil sands. During a conversation I had with a person working in underground coal gasification, it was mentioned that using natural gas to supply heat and steam for extracting bitumen from oil sands was similar to using champagne to make beer.
Overview of the CTL process:
A brief introduction to CTL technology is given in my earlier post on Indian CTL projects. CTL processes produce high quality synthetic liquid fuels (primarily diesel), using coal, oxygen and/or water as raw materials. The best example of a commercial CTL process is the one by Sasol, which produced 7.4 million T of synthetic fuels last year (approximately equivalent to 60 million barrels of oil equivalents, MMBOE). The CTL processes are characterized by high initial capital costs (1-3 billion $ for new plants), large scales (50-100,000 bbl/d plants), and high CO2 emissions. In fact, the Sasol Secunda facility is the largest single-point-source of CO2 emissions in the world. Both direct liquefaction (reacting coal with hydrogen or a hydrogen carrier) as well as indirect liquefaction (generating syngas followed by water-gas shift and Fischer-Tropsch reactions to produce liquid fuels) are possible. The choice is determined by the type of coal, ash content, water availability and other constraints.
The conclusions from the RAND report are given below:
- Basic production costs for SCO are likely to be cost-competitive with conventional petroleum fuels.
- While basic production costs for CTL also appear to be competitive with conventional petroleum fuels across a range of crude-oil prices, CTL competitiveness is more sensitive to technology costs and to oil prices.
- Higher oil prices or significant energy-security premiums increase the economic desirability of SCO and CTL.
- Even with future policy constraints on CO2 emissions and their associated costs, SCO seems likely to be cost-competitive with conventional petroleum; the main potential constraint on SCO production is its local and regional impacts.
- The cost-competitiveness of CTL is more dependent than that of SCO on the costs of CO2 emissions and CCS.
- Unconventional fossil fuels do not, in themselves, offer a path to greatly reduced CO2 emissions, though there are additional possibilities for limiting emissions.
- Relationships among the uncertainties surrounding oil prices, energy security, CCS costs, and CO2-control stringency have important policy and investment implications for CTL.
The C/H ratio of a particular fuel primarily determines the extent of CO2 emissions resulting from producing liquid fuels for transportation (with a generic formula CH2). As shown in the figure (data from "Synthetic Fuels" by Probstein & Hicks), fuels such as bituminous coal with a C/H atomic ratio of 1.2 (or an approximate molecular formula of CH0.8) will need more hydrogen to be added (or more carbon to be eliminated) to make liquid fuels (CH2) compared to heavy crude oil and bitumen (both having C/H ratios of ~0.7).
The disadvantage in extracting bitumen from the oil sands is that it does not flow very well and has to be hydro-treated (with H2) to make it more suitable for processing. As mentioned earlier, the higher CO2 emissions from SCO processes are due to the physical characteristics of the tar sands and not due to the C/H ratio of the raw fuel. This is why the RAND report mentions that SCO processes are likely to be competitive with conventional petroleum, especially in a high-oil price scenario. Moreover, the CO2 emissions from SCO processing is only 25% greater than that for conventional crude oil.
On the other hand, the CTL processes need to have higher quantities of hydrogen (or reject higher amounts of carbon as CO2 to make the hydrogen) compared to SCO and conventional crude oil. These higher emissions are the reason why the economic feasibility of CTL processes has a greater dependence on the costs for mitigating CO2 emissions, compared to SCO.
Labels: beer, bitumen, C/H ratio, champagne, coal to liquids, CTL, economics, RAND, SCO, synthetic crude oil, tar sands, unconventional fossil fuels, upgrading
Penn State My 20 Challenge Week
From the Penn State newswire:
Penn State wants to know, 'What's your 20?'
Penn State is challenging its faculty, staff and students, to reduce electrical consumption by 2 percent during the My 20 Challenge Week of Oct. 19-25. The goal is to reduce the University's energy use by 20 percent and show Penn Staters how it's easy to be environmentally conscious.
Penn Staters are encouraged to find out their carbon footprint though a carbon footprint calculator found at http://www.my20.psu.edu online.
Read the full story on Live: http://live.psu.edu/story/35226/nw63
Related posts: Save energy, save money: Online videos
Labels: carbon footprint, energy conservation, energy efficiency, Penn State
Oct 10, 2008
Economic Value of Nature, Forests
A recent study by Deutsche Bank economist(s) [study leader: Pavan Sukdev] places an economic value on forests based on the benefits they provide like providing clean water and carbon dioxide (CO2) absorption. The EU-commissioned study puts the annual cost of forest loss at between $2 trillion and $5 trillion.
An interesting point of trivia here is that, "Pavan" in hindi/sanskrit refers to the "Wind God".The study, headed by the Deutsche Bank economist, parallels the Stern Review into the economics of climate change.
The study echoes the understanding among some proponents of sustainability that being in harmony with nature is paramount to the survival of species. What this study has done is that it has put an economic value, which provides at least provides a floor to the (much higher) intrinsic value of nature. This is my personal opinion that the intrinsic value of nature is higher than the quoted numbers. If a part of nature were irreparably damaged, then the human economy has to provide them instead, either by carbon dioxide sequestration/conversion, or agricultural production of food and products that were previously naturally produced by forests. A excerpt from the study on the numbers involved compares it to the (smaller) scale of the current economic crisis:
"So whereas Wall Street by various calculations has to date lost, within the financial sector, $1-$1.5 trillion, the reality is that at today's rate we are losing natural capital at least between $2-$5 trillion every year."While you are reading about "natural capital", let me add a note about a book on my wish list: natural capitalism.
The source material for this blog post is the BBC.
Link text: http://news.bbc.co.uk/2/hi/science/nature/7662565.stm
Labels: agriculture, BBC, biomass, carbon, forests, nature, sequestration
Oct 8, 2008
What effects will the current economic downturn have on carbon trading and GHG legislation?
The figure shows how the current downturn (shown as the S&P 500 index, ^GSPC) affects prices for carbon trading (iShares ETF, GRN). Lower energy (oil) futures prices (USO) also have contributed to this reduced demand for carbon credits. Similar trends can also be seen for the actual CO2 price trends on the Chicago Climate Exchange, where the price of a ton of CO2 has fallen sharply to less than 2 $/T. In comparison, the price of first Regional Greenhouse Gas Initiative (RGGI) auction of CO2 emissions last week was 3.07 $/T CO2. As the economy slows down, it seems that people would be willing to pay less for carbon emissions. How this will affect policy is a key question. The McKinsey report (mentioned in an earlier post) notes that cost savings of up to 90 $/T CO2 could be realized by energy efficiency measures in sectors like commercial and residential electronics, and lighting. However, lowering energy prices will lead to lowered incentives for energy conservation. Will either Barack Obama or John McCain have enough political will to pass any carbon regulations? For now, the US presidential candidates seem to be committed to enact GHG regulations, as seen from their last debate.
Related articles:
Projected (2030) greenhouse gas abatement potentials and costs
Article from the Environmental Economics blog
Another article from the Climate Progress blog
Obama's Carbon Ultimatum: from the WSJ
Labels: Barack Obama, carbon trading, GHG legislation, GRN, John McCain, SandP 500, US presidential debate, USO
Sep 27, 2008
Opinion: CO2 to fuels processes
The Green Car Congress blog has an article on a CO2 to fuels process by a company called Carbon Sciences.
1) The use of biocatalysts (enzymes?) to effect the transformations under mild conditions.
2) The use of relatively “dilute” CO2 streams, which could lower the costs for CO2 separation from power plant-flue-gas streams.
My graduate research is in a closely related area, the photocatalytic conversion of CO2 to fuels in which CO2 and water react upon light-induced electron transfer to/from a suitable photosensitizer. This reaction is not very efficient. On the other hand, the heterogeneous hydrogenation of CO2 with H2 is fairly effective (but involves high temperatures), a Japanese company, Mitsui Chemicals will begin the construction of a pilot plant this year to produce 100 T/year of methanol (CH3OH) from CO2 and solar-produced hydrogen.
The conversion of CO2 to fuels is a hydrogenation reaction (add hydrogen, remove oxygen). However, I could not find information on the Carbon Sciences website about their hydrogen source.
One wonders how effective the scale up of this biocatalytic process will be. The main questions for me here are the source of hydrogen, enzyme stability and costs, and the product separation and purification costs. These factors would determine if this process indeed is cheaper than the heterogeneous catalytic process (using Cu/ZnO-like catalysts).
Hat tip: Green Car Congress blog.
Labels: Carbon Sciences, climate change, CO2 to fuels, CO2 utilization, Mitsui Chemicals
Recap:Water: The High Plains Aquifer
The High Plains aquifer underlies one of the most productive agricultural regions in the US. This region is semi-arid and does not get much precipitation, therefore most of the water used for irrigation is drawn from wells. Ground water levels here have been dropping and I thought of doing a zeroth order analysis of how many years will the water in the aquifer last. I used water level change data from USGS. According to USGS, there has been no major change in area under irrigation from 1988-2002. This report also points out that the total change in ground water storage from 1988-2000 was 47 million acre-feet and that the total ground water storage (estimated) in 2000 was 2970 million acre-feet. If we assume that ~30% of this water is recoverable, this gives us an approximate lifetime of 230 years (assuming that the irrigated acreage does not increase). However, the irrigated acreage did increase from 2002-2003, and the rates of depletion are approximately twice their value from 1988-2002. A map showing generalized water-level changes in the aquifer from 2002-03 are shown in the report. As expected, there is a lot of heterogeneity in the way water-levels dropped. Parts of central and south High Plains underwent more drastic water-level changes compared to their northern counterparts. Accordingly, the area-weighted water-level changes per state were more pronounced in Kansas, New Mexico, Oklahoma, and Texas compared to Colorado and Nebraska. Therefore, even if the water might still be around for another 2 centuries, parts of the High Plains might be facing depleted ground water levels sometime soon. These water-level changes might have important implications for US food supply.
Labels: biofuels global warming Science fossil fuels, corn ethanol, High Plains aquifer, Ogallala aquifer, soy bean, US food supply
Sep 17, 2008
Projected (2030) greenhouse gas abatement potentials and costs
Labels: climate change, CO2 abatement, co2 mitigation, CO2 trading, global warming, McKinsey
Sep 12, 2008
Save energy, save money: Online videos
The videos are:
1. Simple energy saving tips: CFLs vs. incandescents, monitoring appliance energy consumption
2. Using less water
3. Environmentally friendly hotels
4. Warm/cold water for laundry
5. Saving money on dishwasher, microwave and oven operation
6. Cutting cooling bills by installing fans and cleaning your AC filters
7. Saving money by doing less paperwork
8. Saving money by borrowing CDs, switching off entertainment centers and reading online newspapers
9. Home energy-audits
10. Eco-friendly interior design
Labels: energy conservation, energy efficiency, energy savings, MoneyTalksNews, save money, Stacy Johnson
Sep 11, 2008
Oil Roundup : 09/11/08
(Disclaimer: This article has no information related to September 9/11 attacks. I plan to do these "oil roundups" more frequently, and the timing was merely coincidental.)
In related news, Petroleo Brasileiro (PBR) also reported a significant offshore medium crude-oilfield discovery, sending the prices of its shares up 6% (on the US markets) while oil was falling. A study by Masters Capital Management found that oil prices were indeed linked to speculation by large financial investors. I do not find anything wrong in speculation; some risk-taking is always good for the markets. However, I will read the report in greater detail to find whether they uncovered evidence for market manipulation.
Summing up, although crude prices seem to be falling because of institutional investors/stronger dollar/weakening oil demand, analysts believe that market fundamentals indicate an upward trend in oil prices.
Previous articles by Nari on price speculation:
Labels: crude oil, Iara, Petrobras, Petroleo Brasiliero, speculation
Sep 7, 2008
Solar Energy and Nuclear Energy in India
Some of the other issues discussed mention the opposition to nuclear plants in India (Koodangulam, in Tamil Nadu) or the resistance to Uranium mines in Kadappa, in Andhra Pradesh. We do see a big similarity here between the environmental permitting process in the US, resistance to Uranium mines in Virginia, the absence of new nuclear plants in past several years.
Virginia is one of just four states that ban uranium mining. The ban was put in place in 1984, to calm fears that had been sparked by the partial meltdown of a nuclear reactor on Three Mile Island outside of Harrisburg, Pa., in 1979.This is an interesting issue because Virginia in US has 2 nuclear power plants and a nuclear submarine/naval command in Norfolk VA. To make a long story short, in many of these cases, there is not enough exchange of information between the authorities and the people who might be in harm's way if a disaster were to occur. Until that exchange of information and dialogues remain inadequate, large scale energy projects would always have big question marks against them.
Note: I have decided to leave this topic a little open ended in order to encourage comments and informed discussion. In addition, in the next several weeks I would be writing more about the prospects for solar and nuclear energy in India.
Labels: concentrated solar, electricity, nuclear energy, solar photovoltaics, subsidies, uranium
Aug 21, 2008
Analysis: How much energy can be realized from waste-to-fuel conversion processes?
This post was motivated by a discussion at Big Biofuels Blog. The question was what impact would these waste-fuel processes make on crude oil imports/consumption.
Current US oil consumption: ~20 million bbl/d
10% of our crude oil use goes towards making petrochemicals. If we assume that 70% of the energy is lost, 1.4 million bbl/d of oil equivalents of energy is still left for us produce fuels (if we do not count the energy that is input while making the petrochemicals). If we use 30% of this waste to make fuel, and assume a (conservative) 15% efficiency for the waste-fuel conversion, we can get 1.4*0.3*0.15 = 0.063 million bbl/d. (or 0.32% of current US crude oil consumption).
To put this in context, transportation accounts for 70% of the current crude oil consumption.
Aug 18, 2008
News: Update on the Vedanta vs. Dongria Kondh case
It should be some consolation to campaigners that nothing Vedanta now does in Orissa will escape notice. Of greater concern are the smaller, more obscure firms which have no reputation to protect. The developing world’s global giants now endure global scrutiny.But not every polluter has upstanding Norwegians investing in it, or holds its AGM in London.
Well put.
Labels: Bauxite mining, India, judgement, Niyamgiri, Supreme Court, Vedanta
Aug 17, 2008
North East Renewable Energy Conference AUg 26-28, Pennsylvania
The 2008 NE Renewable Energy Conference will be held August 26-28 at the Penn Stater Conference Center in State College, Pennsylvania. The conference will showcase regional renewable energy and energy efficiency research, demonstration, and university-industry-government partnerships for sustainable economic development. The audience will be drawn from across the northeastern U.S. and the states of Michigan and Ohio. We are anticipating 300 - 400 attendees from the 14 states in the region as well as Washington, D.C. Sponsors include the Northeast Sun Grant Initiative, the Northeastern Regional Association of State Agricultural Experiment Station Directors, NE SARE, and several other companies and organizations.
Labels: bioenergy, biomass, conference, Penn State, pennsylvania, renewable energy, sustainability
Aug 16, 2008
Analysis: How much biodiesel will jatropha cultivation in UP produce?
From the Green Car Congress blog:
Indian State of Uttar Pradesh to Cultivate jatropha on 40% of Wasteland
16 August 2008
Business Standard. The Indian state of Uttar Pradesh (UP) has set a target to bring at least 40% of its wasteland under jatropha cultivation for biodiesel feedstock within the coming five years.
- The average yield of jatropha is ~1-5 tonnes/ha(from a somewhat dated Frost & Sullivan report on biodiesel). Assuming crude jatropha oil yield of ~2.5-3 tonnes/ha (see above report), UP would produce ~4 million tonnes of crude (jatropha) oil/ha for every crop of jatropha. According to an article in the MIT technology review, 1 hectare of jatropha produces ~1900 liters of "fuel". Therefore, 1.6 million ha. will produce 3040 million liters (800 million US gallons) of fuel for each crop of jatropha (The actual production per year will depend on the number of crops that can be cultivated per year).
- In an other paper, Francis et al. assume a yield of ~580 l biodiesel/ha/year from jatropha cultivation in India. UP would therefore generate ~243 million US gallons of fuel/year (900 million liters of biodiesel/yr).
- What does 240 million US gallons/yr of biodiesel mean ? On a mass basis, it is approximately 0.8 million tonnes of biodiesel/yr. Since the calorific value of petroleum diesel is ~15% higher than that of biodiesel, 0.8 million tonnes of biodiesel would be equivalent to 0.7 million T of petroleum diesel.
- India's diesel "product" imports during 2007-08 were 2.93 million T. (This does not include diesel made from imported crude oil). Therefore, cultivating jatropha in 1.6 million ha. (approximately equivalent to the area of the state of Nagaland or eleven times the area of (the state of) Delhi) will potentially displace ~23% of Indian diesel product imports.
A report on jatropha cultivation in Cambodia, but also has India-specific data.
Labels: biodiesel, crude oil, diesel imports, green car congress, India, jatropha, petro diesel, petroleum diesel, uttar pradesh
Aug 15, 2008
News: BP and Ergo Exergy agreement on UCG
This is somewhat old. Following up from a previous post on underground coal gasification (UCG), BP and Ergo Exergy have teamed together to combine their respective strengths in directional drilling, seismic data interpretation and UCG technologies. The Ergo Exergy website has details of the εUCG™ process.
Labels: BP, coal seam, collaboration, Ergo Exergy, eUCG, UCG, underground coal gasification, εUCG
Opinion: Oil Prices Depend on More on Speculation than assumed previously
By Ann DavisData emerging on players in the commodities markets show that speculators are a larger piece of the oil market than previously known, a development enlivening an already tense election-year debate about traders' influence.
Last month, the main U.S. regulator of commodities trading, the Commodity Futures Trading Commission, reclassified a large unidentified oil trader as a "noncommercial" speculator.
The move changed many analysts' perceptions of the oil market from a more diversified marketplace to one with a heavier-than-thought concentration of financial players who punt on big bets.
Continued... (Graph showing noncommercial positions is courtesy of Wall Street Journal, www.wsj.com)
![[Chart]](http://s.wsj.net/public/resources/images/MI-AR886A_SPECU_20080814184434.gif)
As a result, the number of futures and options contracts held by traders counted as speculators -- those who don't have a commercial need to mitigate the risks of energy prices in their business -- rose to 49% of all crude-oil bets outstanding on the New York Mercantile Exchange, up from 38%.
However in a July 22 release the agency had concluded speculators weren't "systematically" driving oil prices. Oil prices soared until mid-July before beginning a decline. US senators (some Democrats) have questioned the agency's timing of the earlier, incomplete report which painted a different picture. The issue here is that normally the positions taken by hedge funds (who incidentally are not hedging the fuel for any consumption) and other financial firms are in the same direction as the price movement. As far as I know, you cannot short commodities, you can purchase futures contracts.
As I had mentioned previously, additional middlemen are going to drive the price pressure upward. With the release of new data, and with the recent substantial drop in oil prices, the meteoric rise in oil prices over the last 1 year seems to be fueled more by irrational trading demand, rather than organic demand by the new and growing consumers of oil (namely China, Russia, India and Brazil: CRIB, pun intended).
Of course, pure economists and free, free market proponents might still stick to their arguments that oil prices are due to demand, but come on really, stock prices and commodities prices do not reflect their true and fair values on many occasions. A lot of hype (aka " expert opinions) and panic (see below) influence prices on short time scales.
(Cartoon courtesy: http://www.photodarkness.com/blog/astrology/?p=129)
Thankfully over long term, sanity prevails, but who lives for tomorrow, yeah?
Post script: I had to quote Lehman Brothers' opinion, just could not resist!
Lehman Brothers analysts say the CFTC data, as they are now reported, fail to distinguish certain categories of financial traders from commercial traders and create "an opportunity for the activity of less-informed, purely financial investors to distort expectations."
Labels: airlines, cftc, commodity futures, oil prices, speculation, stocks